How to secure drone medical delivery ?
With the new European regulations that came into effect in January 2023, new low-altitude airspace dedicated to drones has been created: U-space. These new airspace areas enable new uses of drones, bringing them closer to populations, cities, and facilitating the transport of goods with enhanced operational security.
It is within this framework that medical delivery by drone can develop, adhering to specific criteria.
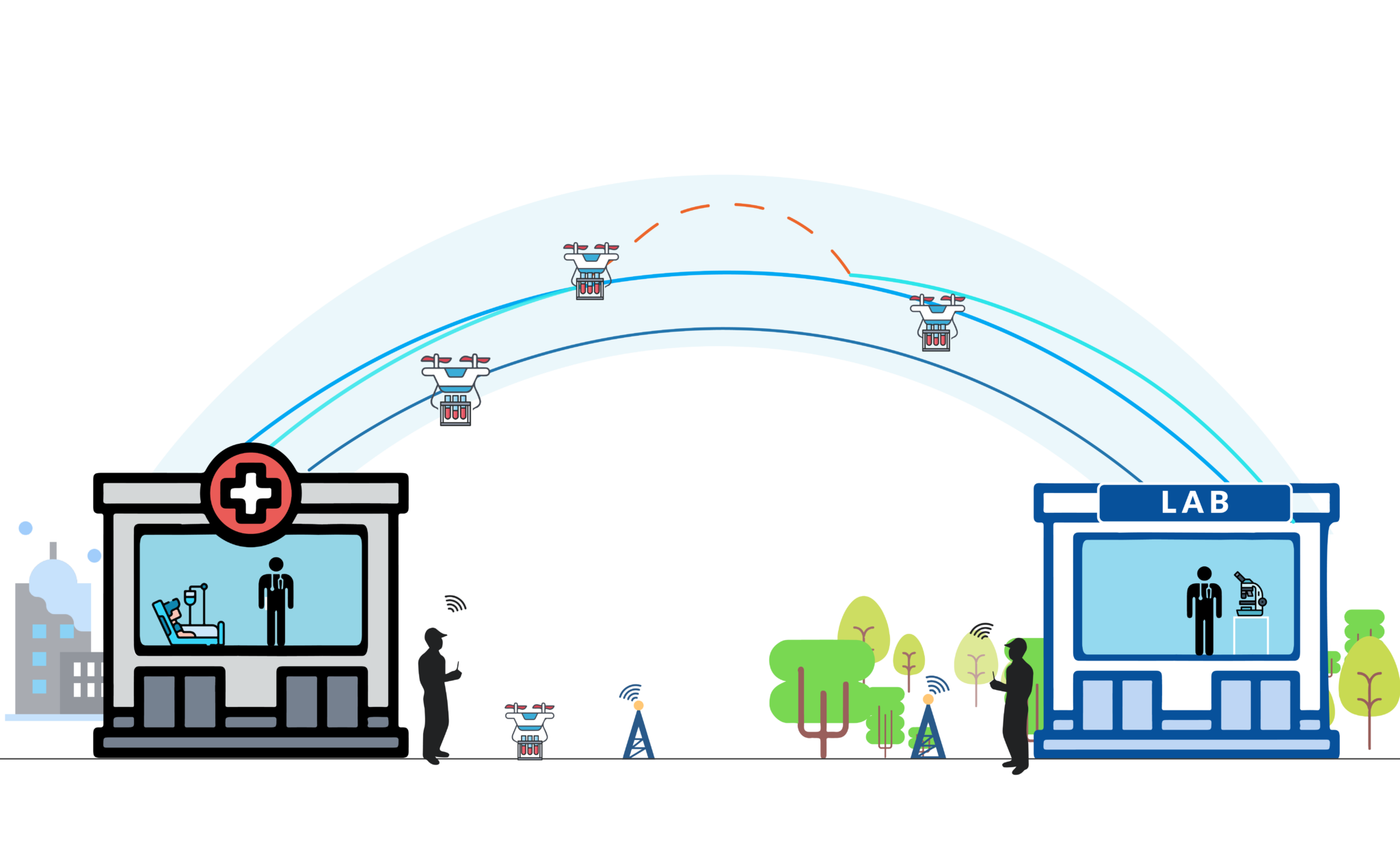
How can the security of a blood sample delivery by drone be ensured?
Drone deliveries, especially of biological samples or medications, adhere to high safety standards.
What are they?
The U-space, or "drone highway," secures the airspace used by drones:
U-space, the airspace in which drones operate, can only be established after numerous security studies and risk mitigation proposals or incident prerequisites are conducted. These spaces are more secure and dedicated to drone traffic than the conventional airspace we are familiar with, managed and secured by a certified private intermediary: the U-space Service Provider.
To create this aerial corridor, the aviation authority of the country (in France, the DGAC) must approve its creation once the file is constructed, and safety evidence is demonstrated.
Once the U-space is created, drone rotations occur through the allocation of time slots, minimizing the risk of collisions.
If a risk is present, the USSP must be able to propose a deconfliction solution through continuous real-time space monitoring.
The security of operations does not end there, as drones used for medical delivery must also meet specific requirements.
What requirements for drones used in medical delivery?
Firstly, delivery operations do not fall within the scope of “standard” operations as defined in scenarios (French or European), this kind of operations belongs to the “certified” operations category.
To operate in this category, drones must be classified according to predefined safety criteria.
Their equipment must ensure the minimization of risks associated with cargo transport, as well as the loss of connection and battery failures, which can occur at any time.
Among various risk mitigation measures, we can notify that drones may be equipped with parachutes.
What protection for biological samples delivered by drone?
Biological samples must be transported while adhering to the cold chain. For this purpose, samples are placed in refrigerated boxes within the drone, with the temperature monitored throughout the journey. Temperature monitoring along the entire route is essential, and the samples are protected with foam reinforcements.
As we have seen, from the creation of the aerial corridor, drone selection, operational criteria, to the protection of the samples, numerous steps ensure the security of medical delivery by drone.
On top of the foam reinforcements, they always are into a triple packaging: a tube, a pouch, a box.
What training for medical employees?
People handling drone deliveries will be trained and authorized in drone management, proper sample handling, and emergency response.
Not only one operator will be required to operate it, but there will be 2 to be able to ensure more safety :
- Security drone operators: Monitoring takeoff and landing, having a safety system, and are able to take control of the drone visually.
- Supervisory drone pilots: The actual pilot who must be trained on the drone and operates it remotely.
This training is given in addition to standard French training “CATT” or European “CATS”.
To conclude
With the regulatory framework as we know it in France regarding drones, these operations of medical delivery by drone, of samples, vaccines, and medications, will be carried out within a strict and secure framework.
To undertake such operations, all risks must be thoroughly understood, and a security solution must be associated with each of them.